Promise of Omics Technologies
Listen to this Article
mins | This voice is AI generated.
mins | This voice is AI generated.
Improve R&D Productivity by Cutting down Risk of Failure and Shorten Timeline
Traditional empiric drug development model with high late-stage attrition of promising therapeutics anticipated declining R&D efficiency
In the last two decades, the changing landscape of drug R&D evidenced significant disadvantages of the traditional empirical drug development model. The empirical drug development model is centric to meet predefined regulatory requirements and getting market access instead of addressing current complex challenges of R&D productivity and providing a value-based patient-centric drug model.a
Historical evidence indicates limitations of the current drug development model to tackle the complex challenges of current drug development. In drug development, the overall success rate from Phase I to regulatory approval has declined to 11.4% in 2018, a significant drop from 14.4% in 2017 and below the average of 14.0% in the prior decade (refer Exhibit 1).
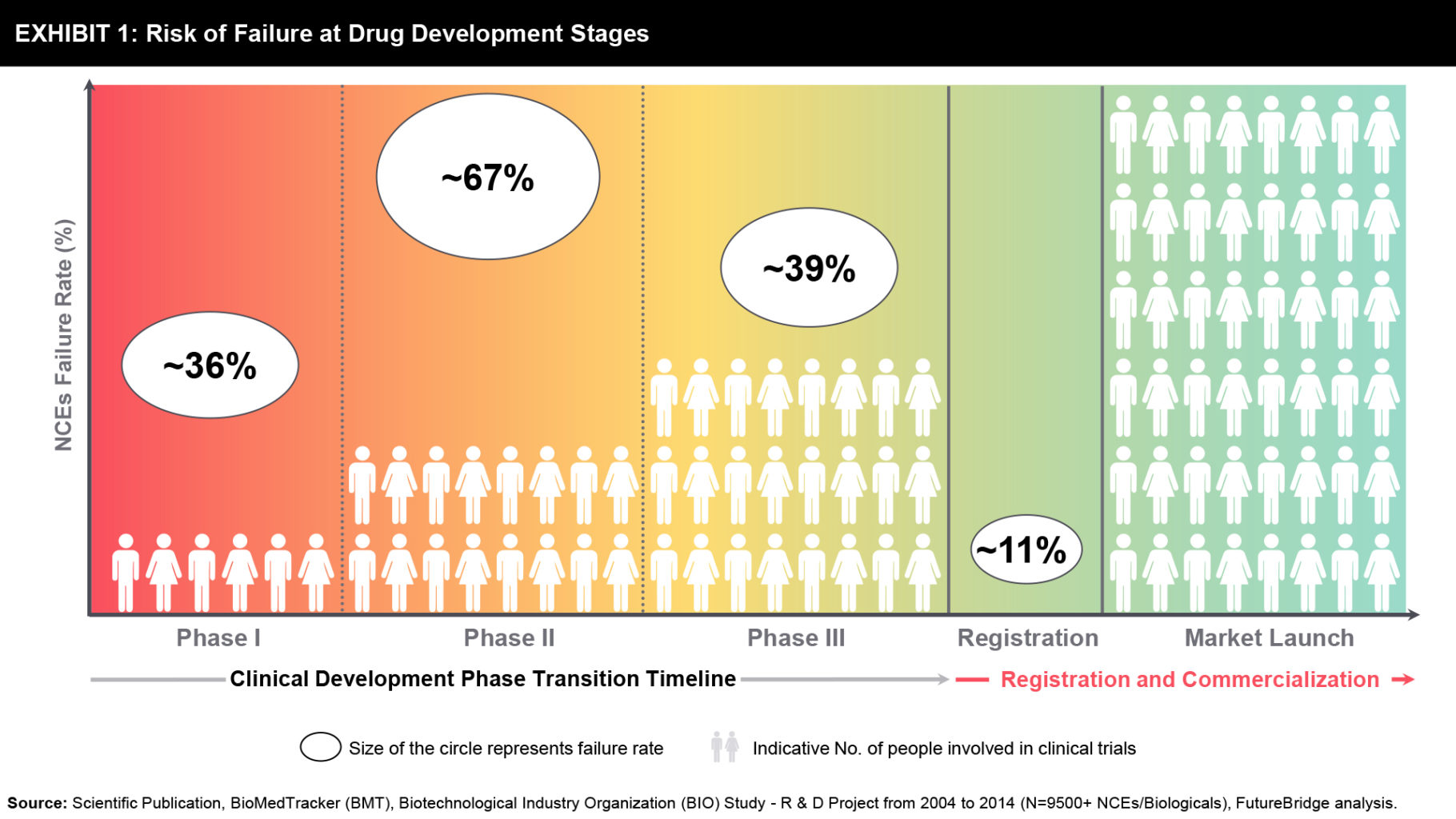

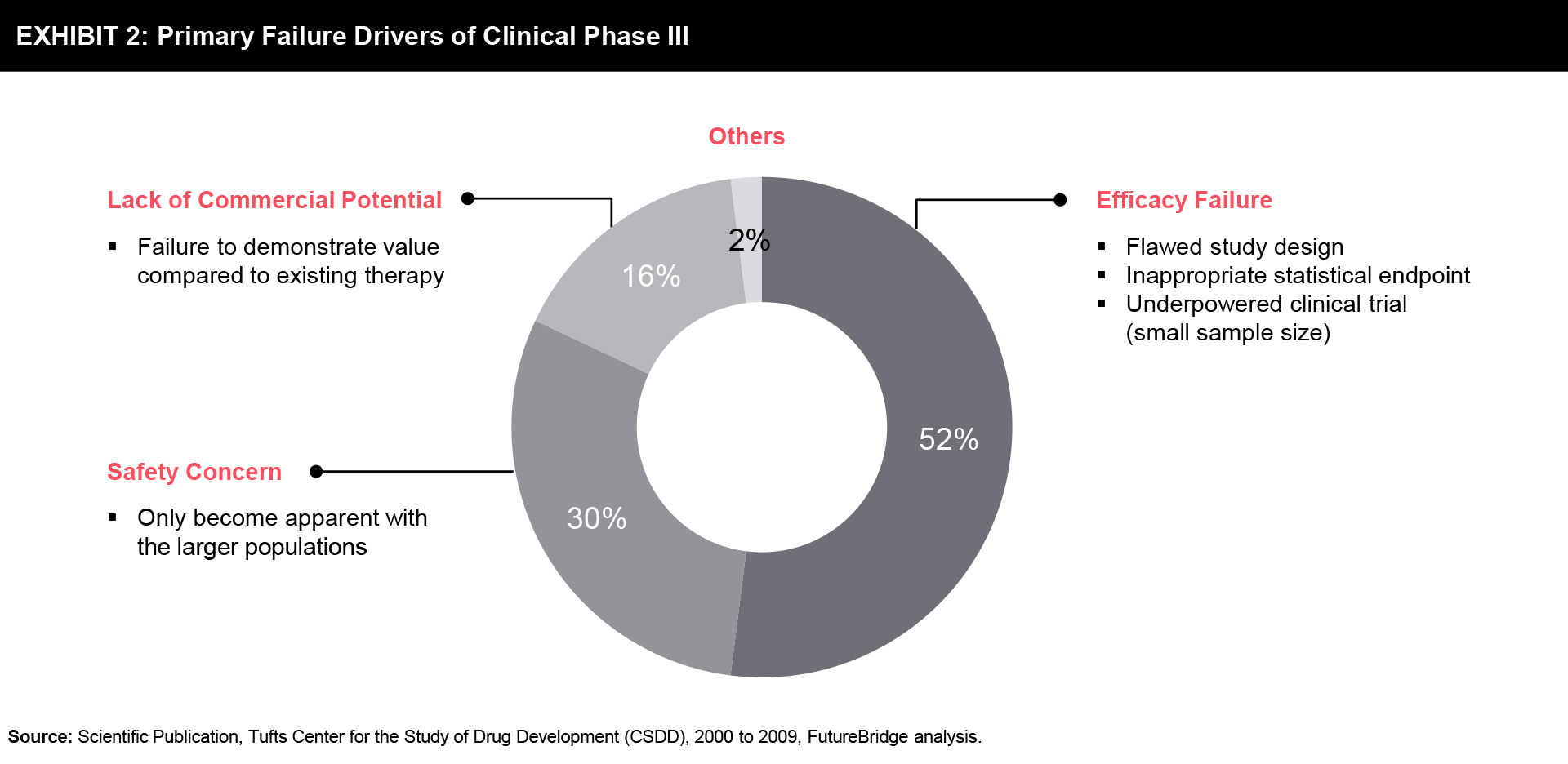
The primary failure rates dominantly attribute to efficacy failure in late-stage clinical phases of drug development followed by patient safety concerns. The lack of superior outcomes to established therapies, a demonstration of commercial potential; insufficient financial budget, and regulatory submission challenges are other emphasized attributes to late-stage attrition of new molecular entities (NMEs) (refer to Exhibit 2).

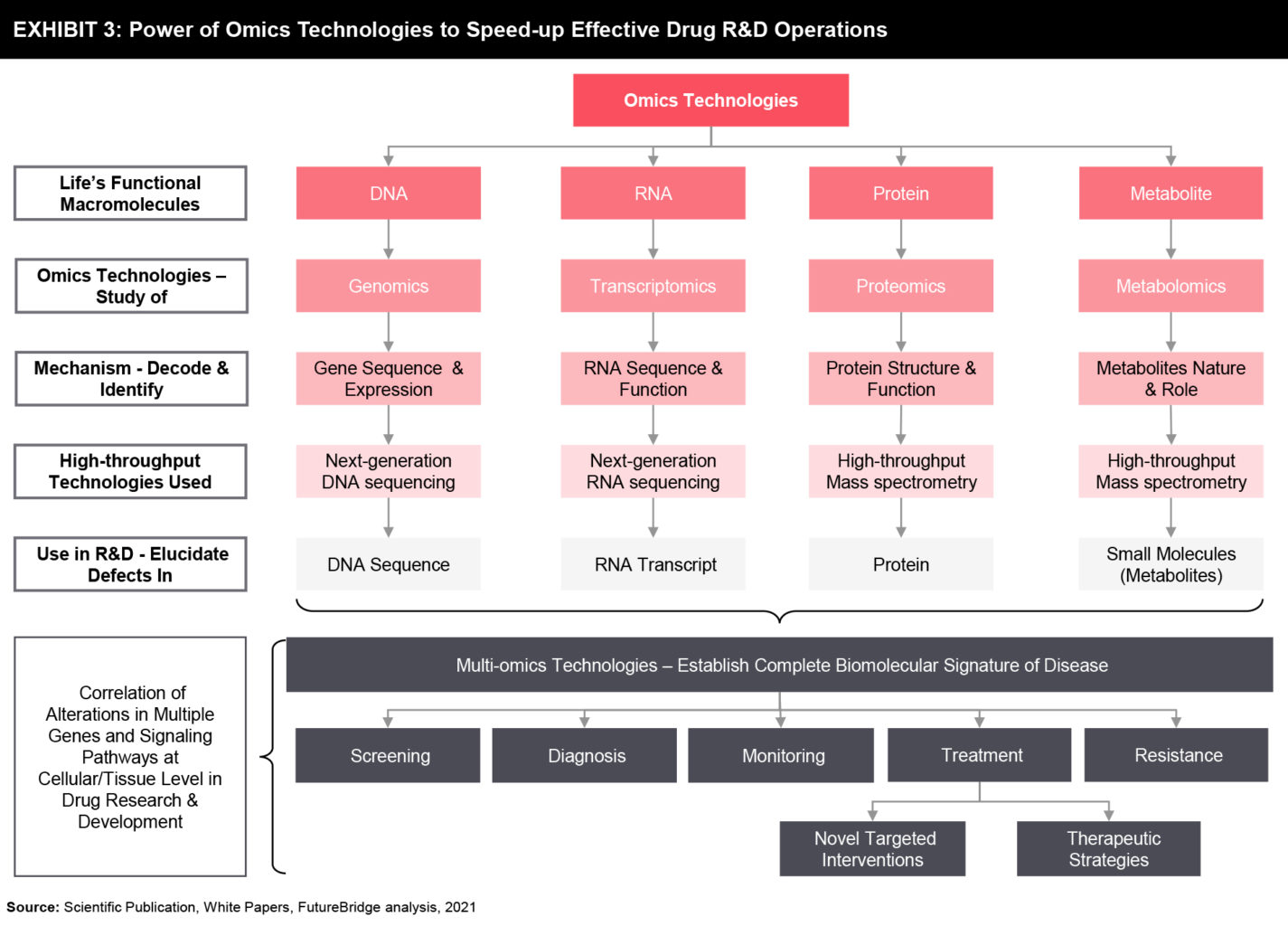
Power of Omics Technologies to Speed-up Effective Drug R&D Operations
“Omics” is derived from -ome, which means “whole.” Omics technologies are simultaneous, high-throughput biomolecular analytical techniques primary includes genomics, transcriptome, proteome, and metabolome to measure cellular level biomolecules such as gene, transcript, protein, and metabolite respectively, and use of these multi-Omics data in R&D to make informed decisions in disease understanding, drug discovery and development (refer Exhibit 3).
The advancements in molecular high-throughput Omics technologies and computational platforms are accelerating precision drug R&D operations. The Omics approach integrates all biological information in systematic ways by using bioinformatics and computational modeling to understand end-to-end biological pathways and identify potential biological molecules at the cellular and tissue levels. Advanced Omics technologies facilitate informed decisions using high-throughput analytical techniques combined with big data bioinformatics to predict the biological interpretation at high speed, accuracy, and reliability.
For instance, genomics technologies can be used in R&D to rapidly elucidate the underlying biomolecular genetic changes associated with specific diseases through a non-targeted approach by a systematic study of a patient’s entire genome. Genomics technologies facilitate rapid and high scale identification, separation, purification, and characterization of variation in genetic biomolecules of targeted disease population. The genomic patient data help in the identification of predisposing genetic risk factors, early drug development through precise emphasis of biochemical pathways linked with disease, the development of accurate genetic biomarker-based screening and diagnostic test, which aid rapid preclinical disease model development and effective stratification of the targeted population in clinical trials. Hence, genomics technologies can accelerate drug discovery and early development processes with the utmost accuracy, precision, and robustness by building a solid foundation for future R&D operations compared to classical techniques.

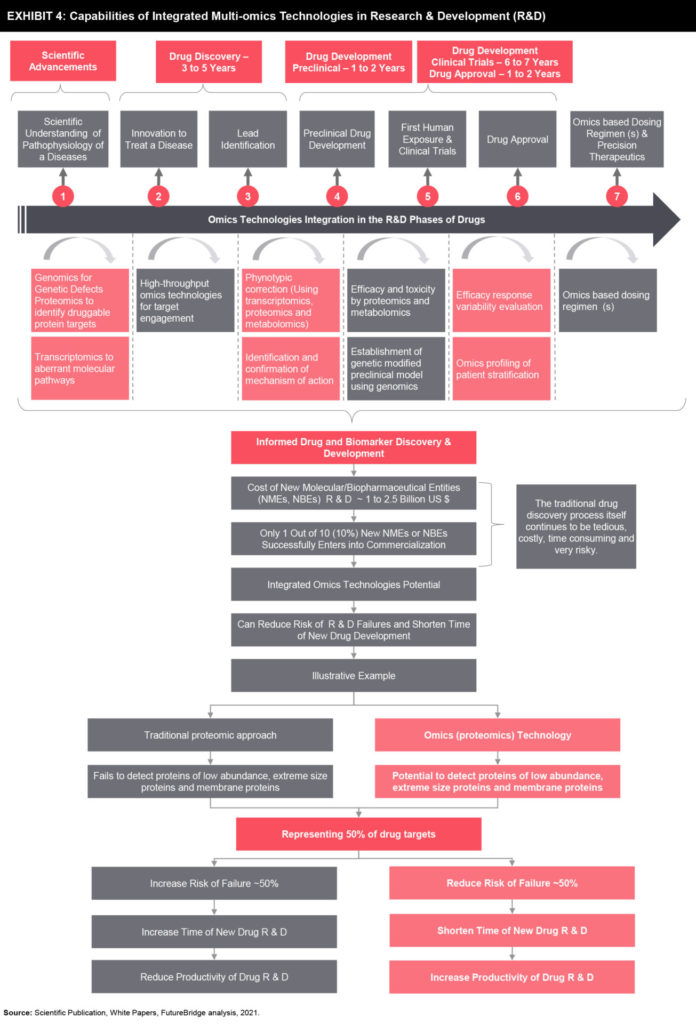
Capabilities of Integrated Multi-Omics Technologies to Transform Classical Pharmaceutical R&D Model
The pharmaceutical R&D is currently experiencing a new paradigm of precision medicine development. The current “one size fits all” approach is expected to replace with targeted and individualized therapeutics, in which disease heterogeneity in the patient is being considered for holistic disease management. However, the classic model of drug development is proving incapable to tackle the emerging unmet needs of personalized treatment development for complex diseases including disease-specific complete biomolecular signature identification and validation of targeted patients.
The traditional analytical technologies used in drug R&D take several years to reveal biomolecular pathophysiology; associated with issues of accuracy, precision, and sensitivity. Classical analytical methods are incapable of providing an integrated and whole biomolecular level understanding approach from ‘gene to a transcript to protein to metabolite.’ This single-angle incomplete approach and incompetent potential of traditional drug discovery and development technologies can pose a significant barrier in the productivity of new drug R&D operations through late-stage identification of efficacy and safety issues associated with the drug candidates. The unreliable and slow method of biomolecular analysis increases the risk of pharmaceutical R&D failures; over-tied financial burden and lengthens the concept to the market timeline of potential novel therapeutic agents.
Multi-Omics technologies are high-throughput methods that purify, identify and characterize biomolecules in complex biospecimen with the complex biomolecular mixture at a tremendously large scale and harness the power of combined biomolecular profiling data to interlink and uncover meaningful biological pathways and biomarkers underlying specific pathophysiology. Effective integration of multi-omic technologies through bioinformatics platforms can facilitate understanding of disease variability, biomolecular heterogeneity of complex diseases, disease progression, and drug resistance mechanisms.
Subsequently, early identification of molecular bio-signature specific to disease and patients can speed up the development of biomarker-based screening, early accurate diagnosis, and effective monitoring techniques as well as personalized treatment approaches. This complete molecular bio-signature allows identification, characterization, validation of drugable biomarker targets and can assist R&D operations ineffective stratification of clinical study population and development of early therapeutic strategies that could turn into highly effective new therapeutic drugs in the future. Hence, multi-Omics technologies can become an essential foundation of the emerging model of drug discovery and development, which is capable of addressing significant challenges of traditional drug development models including lengthy timelines and higher failure rates in R&D of new medicines (refer to Exhibit 4).

An early bidder, Amgen’s Icelandic subsidiary deCODE genetics applying the power of multi-Omics in drug R&D
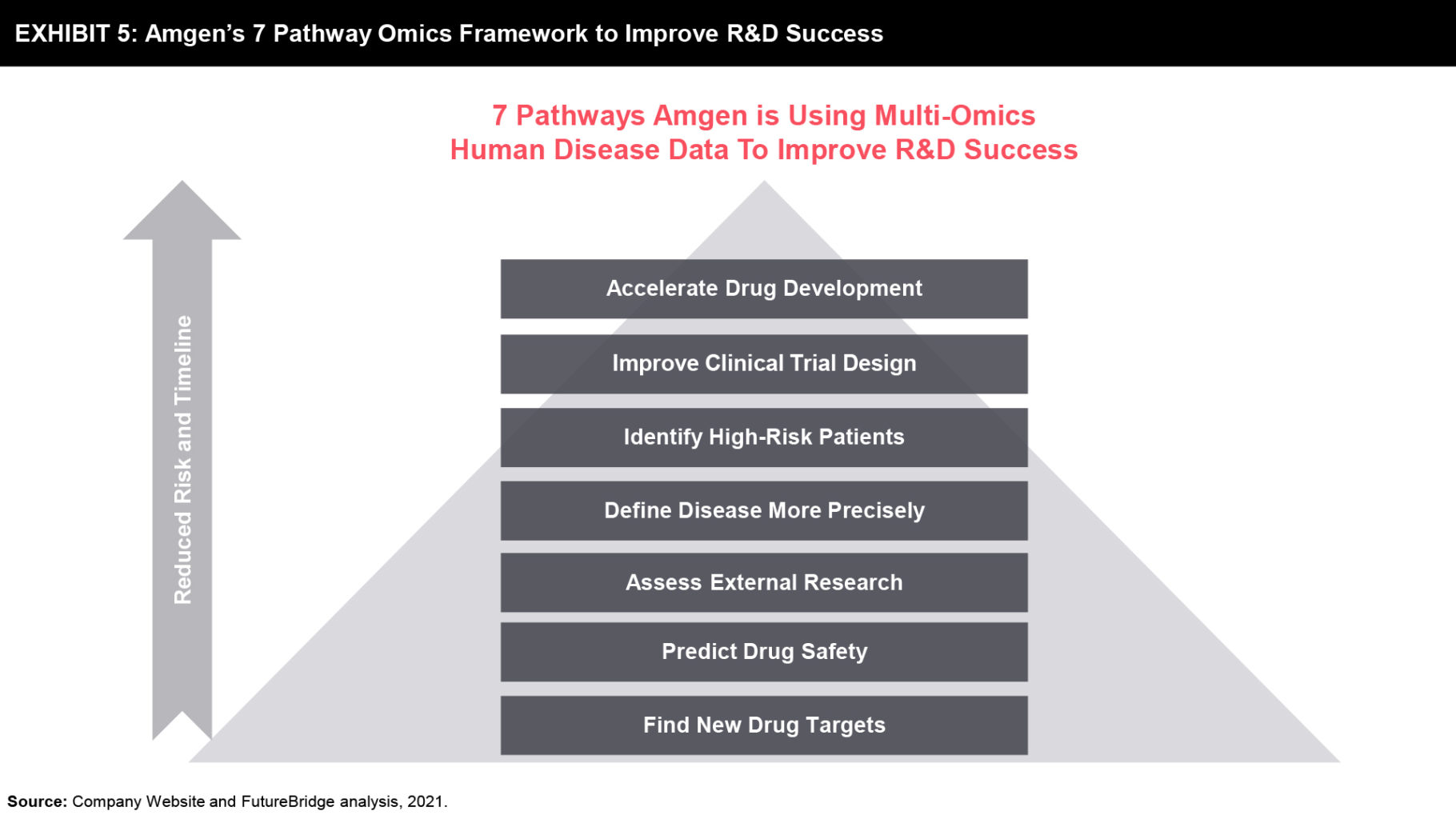
Amgen is amongst the leading biopharmaceutical companies, which is driving and decoding the power of multi-Omics technologies to transform precision medicine drug discovery and development processes since the last decade. Amgen’s 2012 acquisition of subsidiary deCODE Genetics, a pioneer in multi-omics, mining human disease data at an unimaginable scale that was once impossible without multi-Omics technologies. The company has pioneered the concept of large-scale genetic and health data collection and its application to find significant associations between gene relations to disease development. This early decoding of disease development pathways is expected to accelerate the company’s clinical development programs through a noticeable reduction of size and duration of clinical studies. This ensures early high efficacy and safety of NCEs, and speeds up the drug approval process. With a growing data pipeline about the diseases and potential drug targets, Amgen’s R&D is expected to accelerate its pipeline developments with robust efficacy, safety, and novelty (refer to Exhibit 5).

Insilico Medicine’s machine learning analytics platform for analysing R&D Omics data attracting multiple biopharmaceuticals
Insilico Medicine, a Hong Kong-based bioinformatics service provider merges genomics, big data analysis, and deep learning for in silico drug discovery. GlaxoSmithKline and Pfizer have partnered with this ‘service-as-business model’ biotech company to discover novel targets and biomolecules for multiple therapeutic areas. In one of the 2020 deals, Pfizer signed a research collaboration agreement with Insilico Medicine to use its Omics data analytics platform with machine learning technology to invent new druggable targets for numerous pipeline therapeutic areas of Pfizer.
Omics technologies are at the infancy stage in drug R&D operations
In terms of progress, the Omics technologies are in their infancy stages in the R&D of novel medicines and key stakeholders need to strategize their R&D operations to tackle multiple challenges for practical and effective implementation of omics. Omics technologies R&D capability building, operational implementation, data analysis and storage, and sustainable financing to advanced Omics are significant barriers that should be considered by the stakeholders before integrating Omics technologies in their drug R&D processes.
Potential business opportunities of Omics technology in the drug R&D for key stakeholders
Critical business questions to be addressed for Omics application by drug R&D value chain stakeholders:
Share your focus area or question to engage with our Analysts through the Business Objectives service.
Submit My Business ObjectiveOur long-standing clients include some of the worlds leading brands and forward-thinking corporations.




























